WP2 – Catalysts
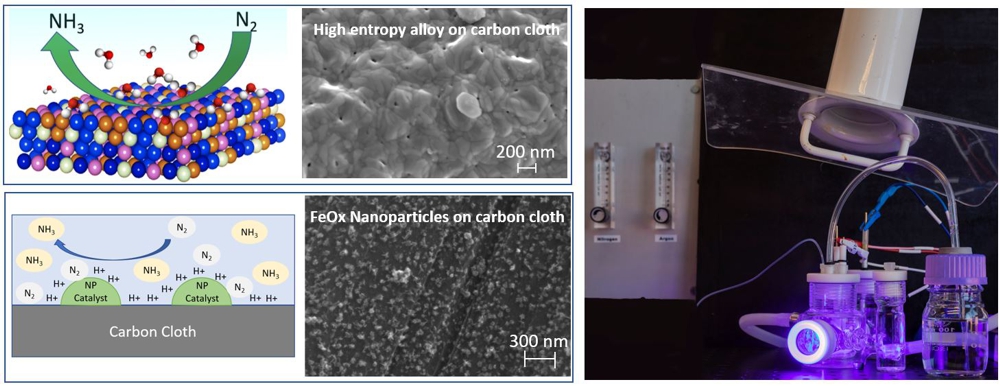
The development of catalysts for electrochemical ammonia synthesis has been focused on two approaches: novel high entropy alloys with five atomic elements (HEAs), developed at Uppsala University, and nanostructured catalysts, developed at IMM-CNR.
Catalysts are deposited on carbon cloth and are tested in a two compartments electrochemical cell (H-cell) as shown on the right of the image.
Ammonia synthesis is obtained at the cathode, feeded with nitrogen gas, through nitrogen reduction reaction. The source of hydrogen is the water based electrolyte.
The glass window allows to perform in operando spectroscopy measurements during the reaction.
Operando Raman spectroscopy and 3-electrode electrochemical measurements setup to study the reaction mechanisms.

WP4 – Electrochemical synthesis of ammonia
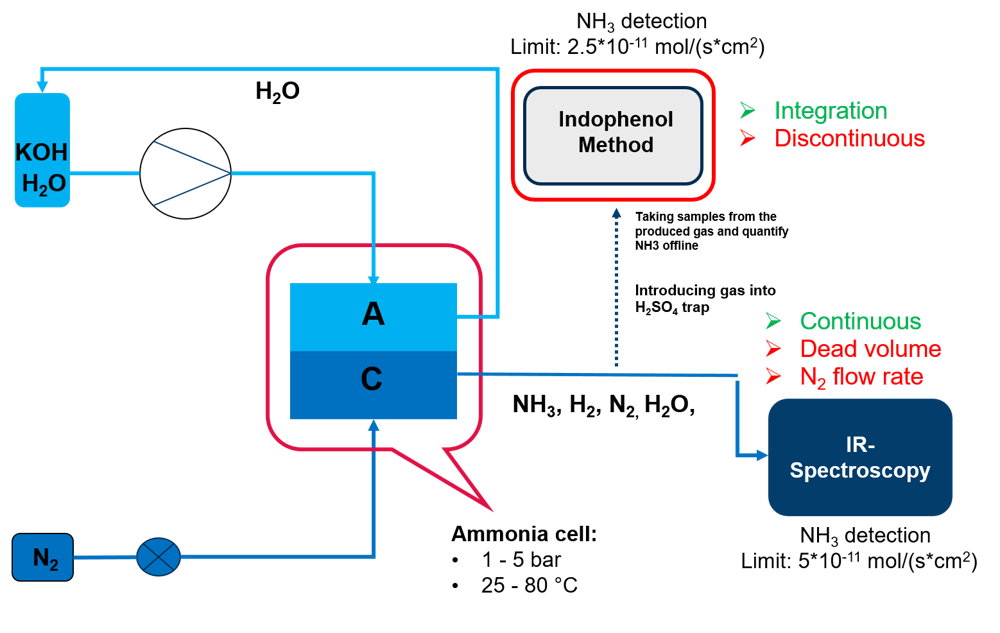
A test facility for the generation of Ammonia from Nitrogen and aqueous electrolyte was set up and ammonia detection by IR-spectroscopy and the indophenol method was integrated.
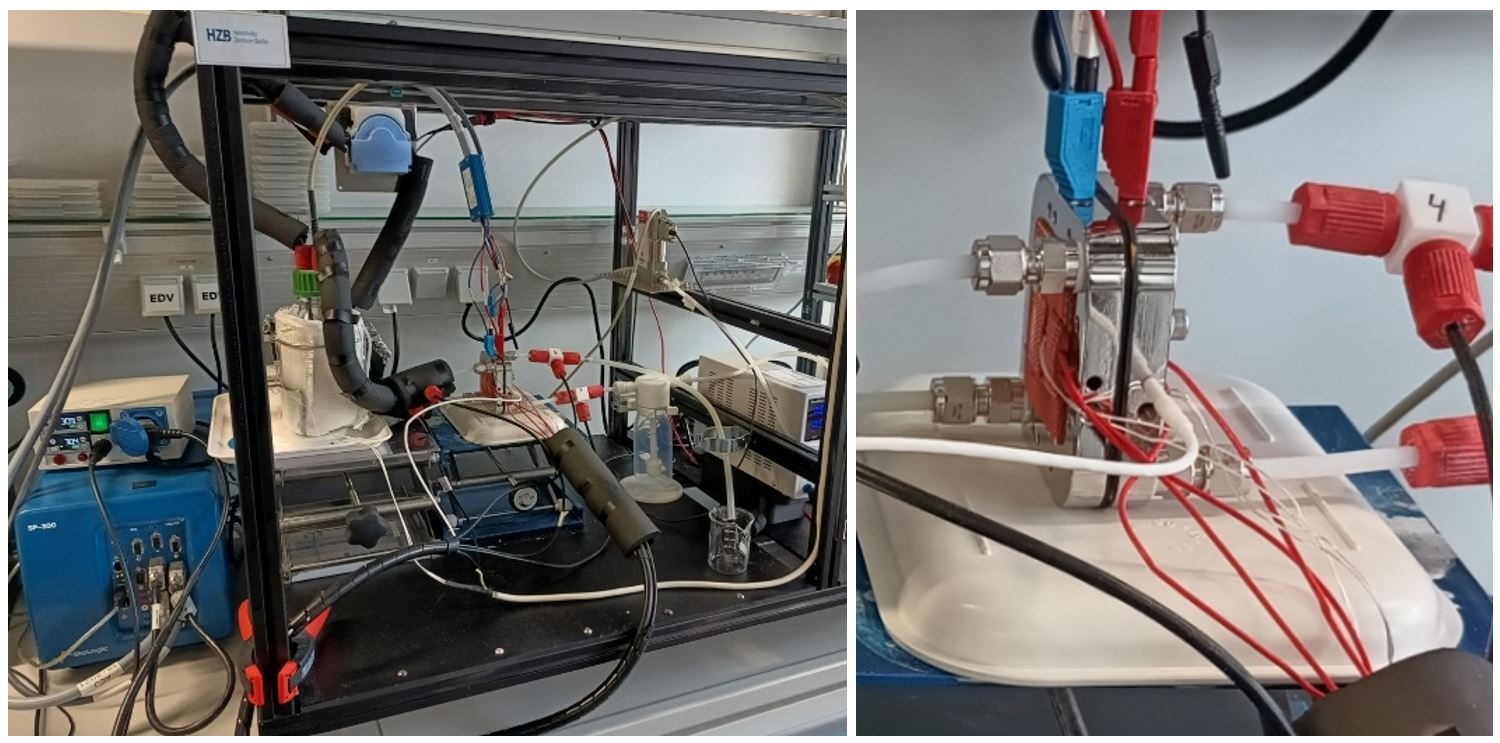
WP5 – Direct Ammonia Fuel Cell
DAFC measurement setup, including the second generation of the test cell with heating pads, electric and fluid connections, and a thermometer to monitor the plate temperature. The cell has been designed and manufactured at HZB using catalysts developed within the consortium, with Pt group metal loading lower than 0.05 mg/cm 2.
WP6 – Green Ammonia cycle
For the realization of the full green ammonia cycle a test bench has been built at FZJ using a source measure unit. It is capable of emulating solar cells for direct coupling experiments reliably, accurately and independently from weather conditions. It will be employed to evaluate the devices under fluctuating power conditions.




 Visit Today : 114
Visit Today : 114 Total Visit : 124487
Total Visit : 124487 Who's Online : 1
Who's Online : 1