Ammonia is one of the most important chemicals but its production requires an energy intensive process, responsible for about 1-2% of total CO 2 emissions worldwide.

Ammonia is also potentially a formidable energy vector, with large hydrogen content, high energy volumetric density and, unlike H 2, ease of liquefaction for storage.
The main objective of TELEGRAM is to demonstrate, at the laboratory scale level, a complete green ammonia carbon–neutral energy cycle, based on electrochemical processes, enabling the use of ammonia as a green fuel.
Achievement of this target requires the development of two key enabling technologies.
Electrochemical ammonia synthesis.
This will be developed by adopting a multi-stage membrane reactor which, starting from air, water and renewable sources (sunlight or wind), will produce ammonia at temperature <100°C.
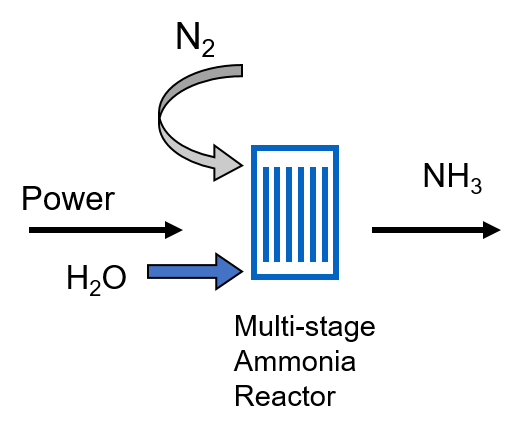
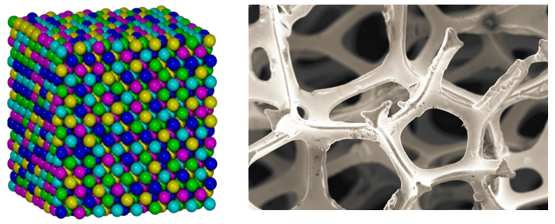
Novel materials, such as high entropy alloys and nanostructured catalysts will be studied and implemented in the reactor.
The objective is to reach performance values able to make the process effective for industrial exploitation, i.e. faradaic efficiency >50% and production rate of at least 0.1 µmol/s/cm 2.
Direct ammonia fuel cell (DAFC).
For DAFC the best catalyst choice is platinum group metals (PGMs).
The objective is to achieve a power density of at least 100 mW/cm 2 and chemical to electricity efficiency >25%, with PGM loading <0.05 mg/cm 2 while operating below 100°C.

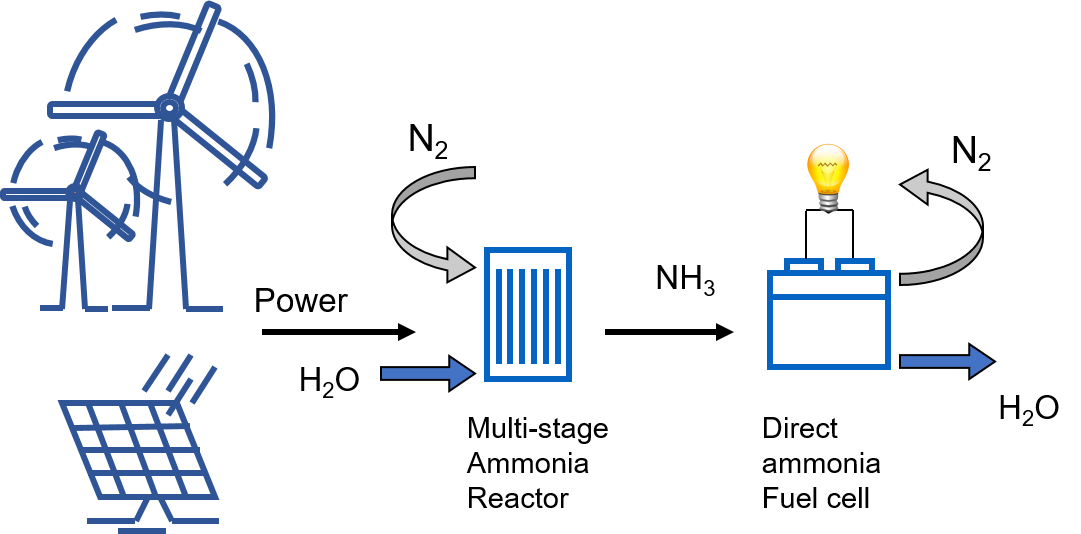
The developed ammonia reactor, powered by renewables, and the DAFC will be coupled together to demonstrate the complete ammonia energy cycle at a laboratory scale.
The objective is to achieve 95% of the combined efficiencies of ammonia generation and fuel cell. This result will contribute to establish an European innovation base on the two key enabling technologies and on novel catalysts, and to build a sustainable renewable energy system.



 Visit Today : 101
Visit Today : 101 Total Visit : 81045
Total Visit : 81045 Who's Online : 2
Who's Online : 2